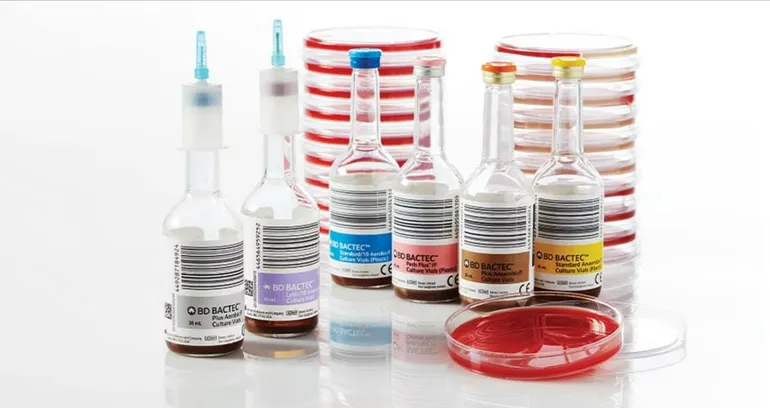
FDA Cautions That Shortage of BD Blood Culture Bottles May Affect Diagnostic Accuracy
In a recent announcement, the Food and Drug Administration (FDA) has raised concerns about disruptions in the supply of BD Bactec blood culture media bottles, highlighting potential impacts on patient diagnosis and subsequent management. Becton Dickinson (BD), the manufacturer of these bottles, acknowledged in June that its customers might face “intermittent delays” in receiving supplies […]