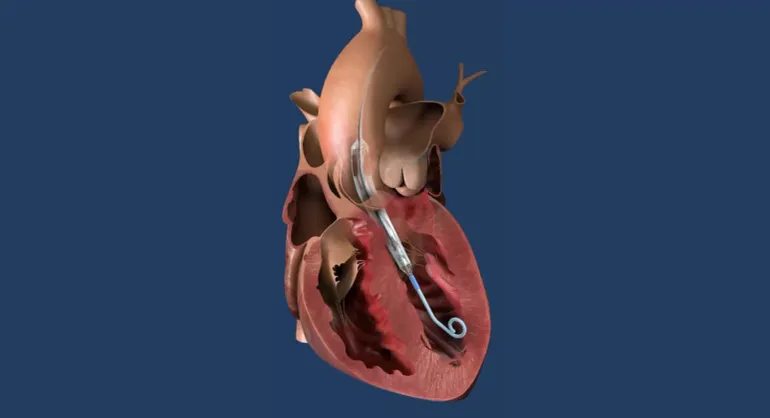
The Future of Psychedelic Medicines: What Lies Ahead?
At the HLTH 2024 conference in Las Vegas, experts discussed the future of psychedelic medicines following the FDA’s decision to delay the approval of MDMA-assisted therapy for PTSD. This setback didn’t mark the end of advancements in the field, as emphasized by various professionals during a panel discussion. Lykos Therapeutics had submitted a new drug […]