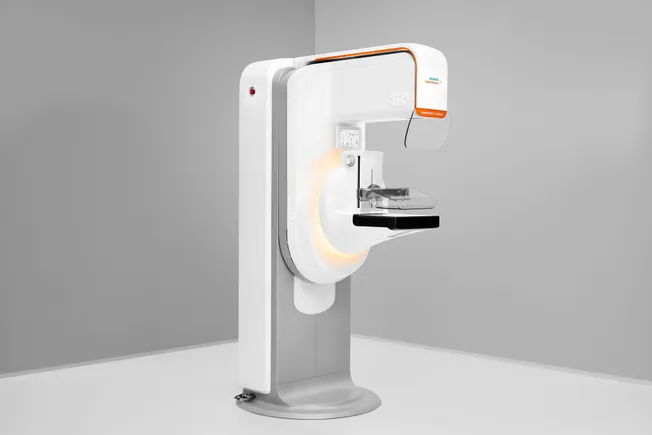
Siemens Healthineers has recently received premarket approval from the FDA for the 3D imaging component of its newly redesigned mammography system, the Mammomat B.brilliant. This marks the company’s first major overhaul of its mammography platform in more than a decade. The approval highlights a significant advancement in the use of 3D imaging technology to enhance breast cancer screening and diagnosis.
The Mammomat B.brilliant system incorporates innovative 3D image acquisition and reconstruction technologies that address the limitations presented by traditional 2D mammography, such as overlapping breast tissues, which can obscure the visibility of lesions. By implementing wide-angle technology, which Siemens claims to include the widest available angle of 50 degrees, the system improves lesion detection and microcalcification identification within the breast tissues. This technology facilitates a more accurate assessment of breast abnormalities, potentially leading to earlier and more reliable breast cancer detection.
One of the key advantages of the Mammomat B.brilliant system is its ability to produce high-resolution 3D images swiftly. The system boasts a scanning time of about five seconds, which is approximately 35% faster than what other similar devices on the market offer. This efficiency not only enhances the imaging process but also improves the overall patient experience by reducing the time spent under discomfort during the scanning process.
Additionally, several user-centric improvements have been incorporated into the new system compared to its predecessor, the Mammomat Revelation. These enhancements focus on patient comfort, user workflow, and ergonomic design. For example, during image acquisition, patients have the ability to lean into the system, which provides greater stability and comfort. These improvements reflect Siemens Healthineers’ commitment to enhancing both the functionality of their mammography systems and the satisfaction of the end users, including patients and healthcare professionals.
The recent FDA approval of the 3D imaging capabilities of Mammomat B.brilliant coincides with the implementation of updated regulations under the Mammography Quality Standards Act (MQSA). As of September 2023, these regulations require mammography facilities to be diligently certified and accredited, while also mandating periodic reviews of clinical images to ensure quality and compliance. Furthermore, the updated regulations now require facilities to inform patients about their breast density following a mammogram. High breast density not only complicates the detection of cancer with mammography but also increases the risk of developing breast cancer itself. These regulatory changes underscore the increasing importance of advanced diagnostic tools like the Mammomat B.brilliant in improving cancer detection rates and patient outcomes.
Siemens Healthineers’ introduction of the Mammomat B.brilliant system represents a significant leap forward in mammography technology. By leveraging advanced 3D imaging capabilities and wide-angle technology, the system sets a new standard for accuracy and efficiency in breast cancer screening. The timing of this rollout aligns well with recent regulatory changes aimed at enhancing the quality of mammography services and ensuring patient safety. As Siemens continues to innovate in the medical imaging field, healthcare providers will be better equipped to deliver superior care and outcomes for patients facing breast cancer risks.
#Siemens #Healthineers #wins #FDA #approval #mammography #system