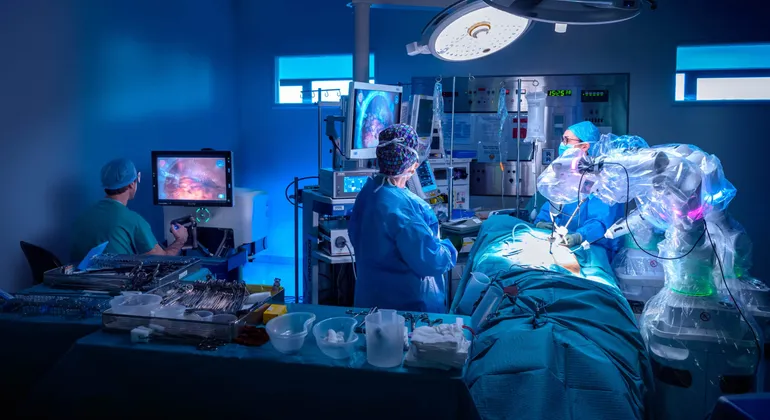
CMR Surgical recently celebrated a significant achievement by obtaining FDA authorization for its Versius robotic system, initially approved for gallbladder removal surgeries. This milestone marks the first FDA approval granted through the agency’s de novo pathway for a multi-port, soft tissue general surgical robot. The de novo pathway helps introduce new medical devices into the market, potentially serving as predicates for future 510(k) submissions.
The news came shortly after CMR Surgical appointed Massimiliano Colella as the interim CEO, taking over from Supratim Bose who resigned for personal reasons. Colella, previously the chief commercial officer at CMR, has stepped into leadership during a pivotal moment for the company.
Founded a decade ago and based in Cambridge, U.K., CMR Surgical has positioned itself as a strong competitor in the global robotic surgery market, aiming to challenge the current market leader, Intuitive Surgical. CMR’s Versius system, noted for its compact and versatile design, was designed to be sufficiently adaptable to operate across various care settings, potentially democratizing the availability of robotic-assisted surgical procedures.
Versius had already secured Europe’s CE mark in 2019 and has become the second-most-used robotic surgical system globally, outside of the U.S. The robot has been utilized in over 26,000 surgeries across different regions including Europe, Latin America, Asia, the Middle East, and Africa. With FDA clearance now secured, Versius extends its reach into the U.S. market, which CMR views as crucial for its strategic growth. The robotic surgery market in the U.S. is seen as underpenetrated; a study highlighted that only about 2.5% of the nearly 10 million major operating room procedures conducted in 2017 in the U.S. utilized robotic assistance.
Erik Wilson, vice chair of surgery at the University of Texas McGovern Medical School at Houston, commented on the FDA authorization, recognizing it as a significant advancement in enabling hospitals of any size to offer robotic-assisted surgeries. He emphasized the potential of Versius to make minimal access surgery more accessible to a broader range of patients.
To propel its entry and expansion in the U.S., CMR has strategically refreshed its leadership team. This includes the appointment of Daniel Moore, former chairman of Livanova, as the non-executive chairman of CMR’s board. Additionally, Markus Bauman joined as chief legal and business affairs officer, and Michelle Paknad was named senior vice president for global business development.
Financially, CMR Surgical is well-supported, having raised significant capital to fund its ambitions. The company secured $165 million in financing last year, adding to a substantial $600 million funding round in 2021. These financial injections underscore the confidence investors have in CMR’s strategic direction and its potential to make a significant impact in the global surgical landscape.
In summary, CMR Surgical’s FDA authorization for its Versius robotic system represents a transformative step for the company in its mission to expand the accessibility of robotic-assisted surgeries worldwide. By entering the U.S market and strengthening its leadership and financial standing, CMr Surgical is well-positioned to capitalize on the opportunities within the evolving landscape of surgical technology.
#CMR #Surgical #FDA #novo #nod #Versius #robot