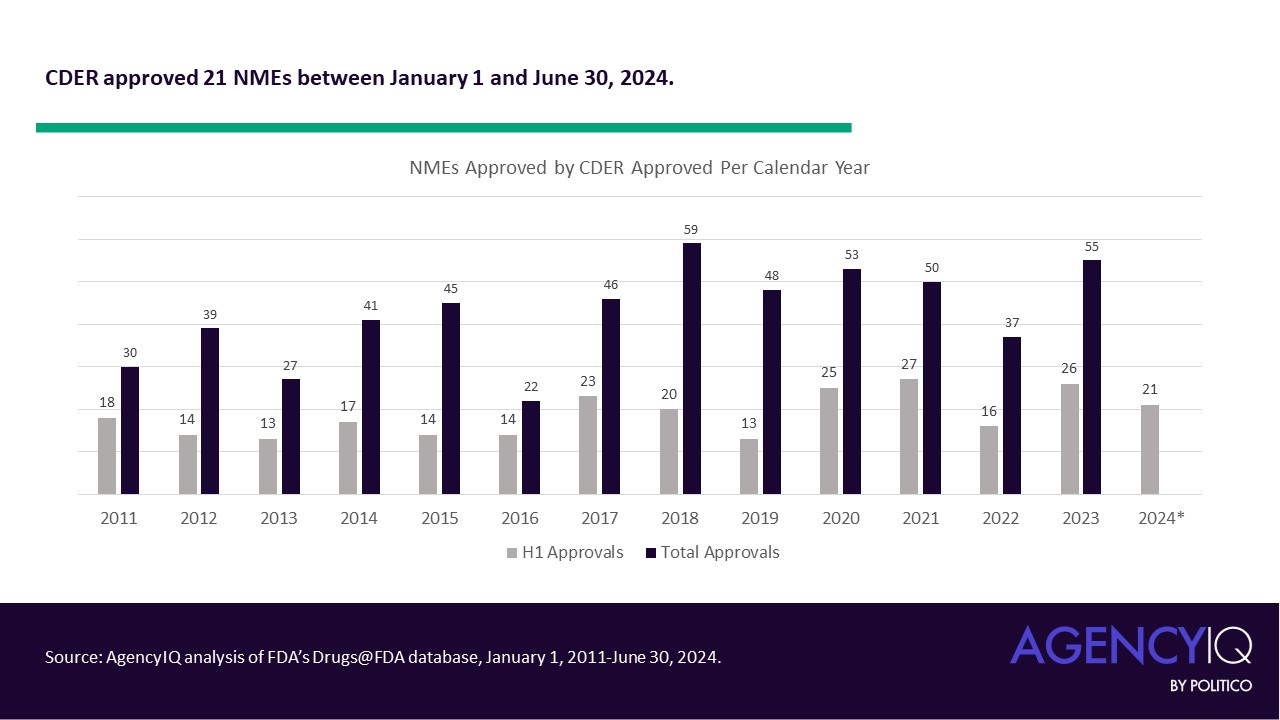
FDA approves Guardant’s blood test for colon cancer
The Food and Drug Administration (FDA) has recently approved Guardant Health’s Shield blood test, marking it as a primary screening tool for colorectal cancer for average-risk adults aged 45 and older. This approval positions the Shield test as a more appealing and convenient alternative to traditional colorectal cancer screening methods, such as colonoscopies and stool-based […]