Otsuka Precision Health Introduces First Digital Therapeutic for Depression
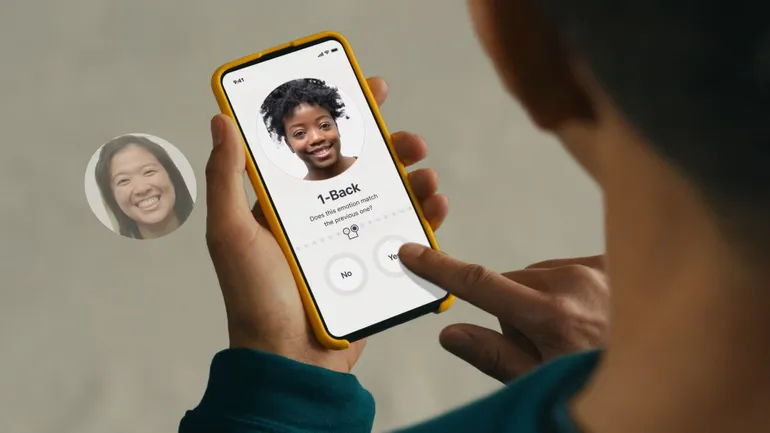
Otsuka’s digital health subsidiary has launched Rejoyn, an innovative app-based treatment specifically developed for managing major depressive disorder (MDD). Available solely on prescription, Rejoyn complements existing medication treatments and features a six-week program that uses principles of cognitive behavioral therapy. The program includes video lessons and exercises to assist users in recognizing and managing their […]